In the pursuit of combating Alzheimer’s disease, a new study sheds light on some existing medications that could be game-changers. Researchers have identified two cancer treatment drugs that show considerable promise.
The drugs in question are letrozole, primarily used in breast cancer therapy, and irinotecan, which targets colon and lung cancers. Since these drugs are already approved by US regulators, clinical trials for their use in Alzheimer’s could commence sooner than expected.
A team from the University of California, San Francisco (UCSF), along with Gladstone Institutes, investigated the changes that Alzheimer’s brings to gene expression in the brain. They utilized a medical database known as the Connectivity Map to search for substances capable of reversing these gene changes and cross-referenced with patient records from cancer treatments, discovering that those who took these drugs had a lower risk of developing Alzheimer’s.
“Alzheimer’s disease involves complex modifications in the brain, making it challenging to address, but our computational tools allowed us to tackle this complexity head-on,” expressed computational biologist Marina Sirota from UCSF. “We’re thrilled that our innovative approach has led us to consider existing FDA-approved medications for potential Alzheimer’s treatment.”
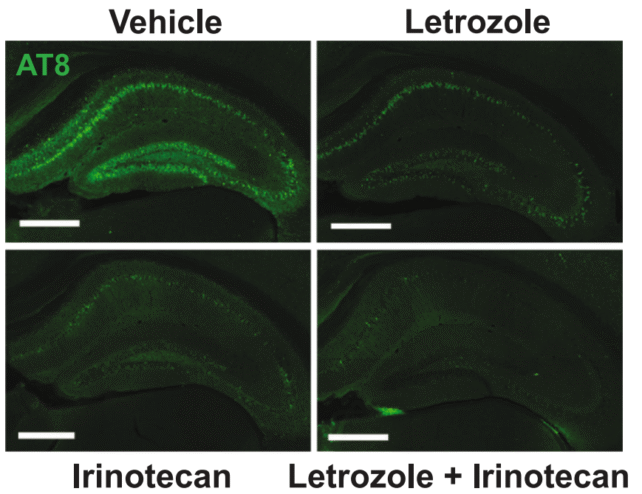
After pinpointing letrozole and irinotecan as key players, the team tested these drugs on mouse models afflicted with Alzheimer’s. The dual-treatment approach began to reverse various detrimental brain alterations caused by the disease.
Remarkably, the accumulation of harmful tau proteins in the affected brains was significantly reduced, and there were noticeable improvements in learning and memory tasks—capabilities often hindered in individuals facing Alzheimer’s.
By leveraging both medications in tandem, researchers effectively targeted different brain cell types affected by the disease. While letrozole appeared to counteract Alzheimer’s within neurons, irinotecan provided support in the glial cells.
Neuroscientist Yadong Huang, from UCSF and Gladstone, noted, “Alzheimer’s likely stems from various changes to a multitude of genes and proteins, creating disruptions in brain health. This makes drug development quite challenging, as conventional methods typically deal with single entities.”
While this research heralds a promising breakthrough, further work is necessary. As of now, these drugs have only been tested directly in mice, and their potential side effects must be considered if they are repurposed for Alzheimer’s treatment.
The next logical step involves initiating clinical trials for human patients diagnosed with Alzheimer’s disease. This could pave the way for treatments that are not only personalized but also more effective, based on how gene expression has varied in each instance.
Currently, more than 55 million people globally have Alzheimer’s, and projections suggest that this number could double over the next 25 years as the population ages. Therefore, finding preventative measures or ways to reverse symptoms will dramatically impact global health.
“If independent data sources, such as single-cell expression details and clinical records, reveal similar pathways and drugs, and then effectively address Alzheimer’s in genetic models, then we might be on the right track,” Sirota stated optimistically. “We’re hopeful this could lead to genuine solutions for millions impacted by Alzheimer’s.”
The findings are documented in Cell.



















