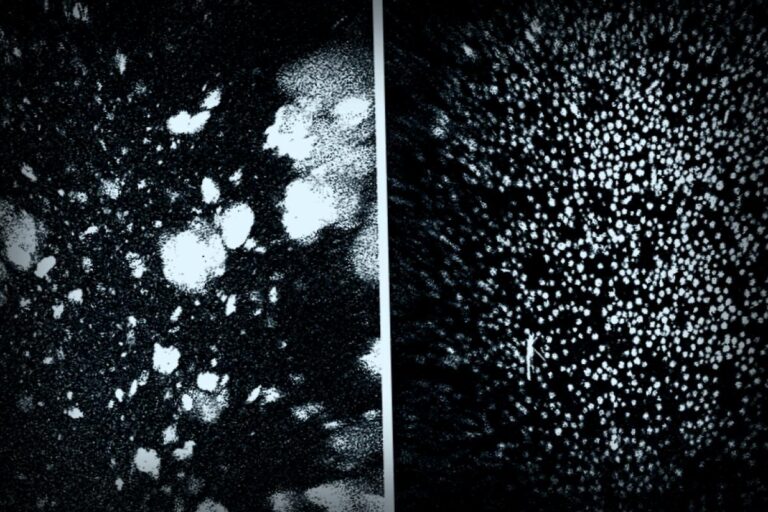
For years, Alzheimer’s has felt like an impregnable stronghold. Scientists have relentlessly experimented with various methods to target beta-amyloid proteins—sticky clumps that signal the presence of this challenging disease. However, most conventional medications attempting to combat these plaques have produced underwhelming results.
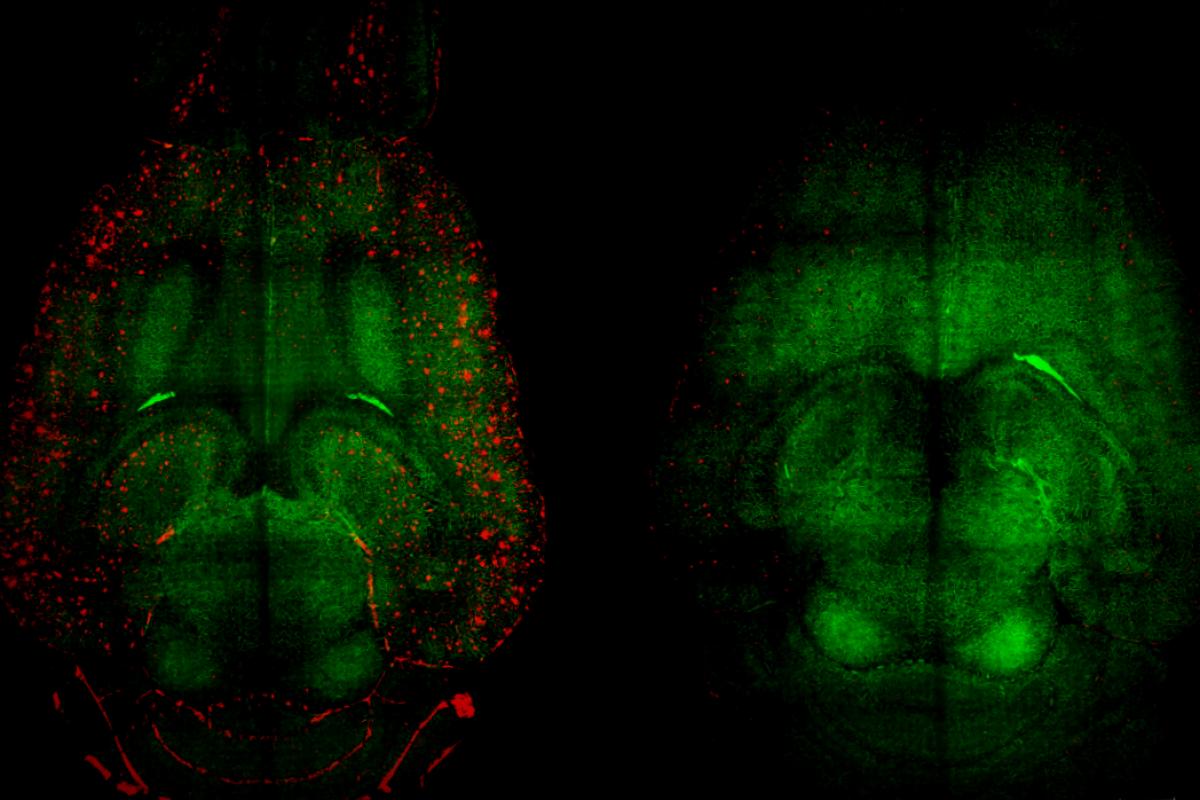
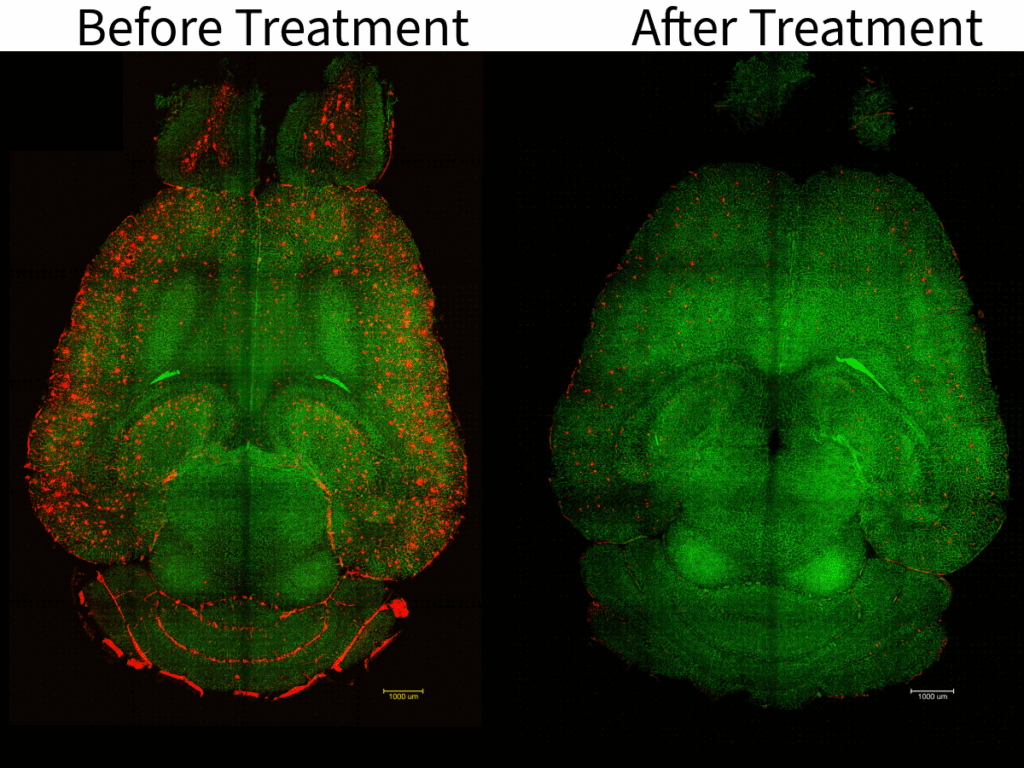
But now, in a remarkable turning point, a group of international researchers has taken a completely different approach. Instead of merely trying to eliminate the plaques, they focused on rejuvenating the brain’s natural waste-clearing system. Through the use of nanoparticles, likened to a clever “Trojan horse,” they successfully restored the essential functions of the blood-brain barrier (BBB). In a mere two hours, they managed to eliminate almost half of the amyloid plaque from the brains of mice suffering from severe Alzheimer’s.
The recovery observed not only resulted in immediate results; the cognitive improvements lasted. Mice that underwent treatment experienced memory and learning capabilities akin to those of their healthy counterparts, with noticeable benefits persisting for at least six months post-treatment.
Cleaning Up the Mess
To understand the mechanics of this groundbreaking approach, we must first delve into the role of the blood-brain barrier. The brain, highly delicate and sensitive, requires robust protection to keep toxins and pathogens at bay. The BBB functions almost like a strict bouncer, screening every molecule while only permitting vital nutrients to enter.
However, this bouncer has an additional essential task: it also helps dispose of harmful waste.
In a properly functioning brain, the BBB plays a crucial role in clearing away amyloid-beta (Aβ), a natural byproduct of normal brain activities. When everything is in order, this protein gets eliminated. In conditions like Alzheimer’s, though, this waste clearance breaks down. The Aβ accumulates, forming clumps that incite inflammation, hamper neuron communication, and eventually lead to cell death.
Traditionally, scientists believed that the decay of the BBB was just a symptom emerging later in the disease’s advancement. However, accumulating research suggests it may actually be one of the initial failures—serving as a trigger for the debilitating chain reaction.
“Most current Alzheimer’s therapies aim to remove amyloid-β or offer protective measures after damage has already occurred . Yet by that point, the brain’s protective barrier is compromised and struggles to deliver nutrients or clear waste effectively. Our focus is on restoring the barrier as it lies at the heart of the issue. A well-functioning BBB supports brain cells, manages inflammation, and maintains a healthy environment for neuronal function,” says Giuseppe Battaglia, one of the researchers, in correspondence with ZME Science.
The Perfect Fit
Repairing the blood-brain barrier is no small feat. With around a billion tiny capillaries, it comprises a highly intricate system. A key element here is a protein known as LRP1 which helps eliminate toxic amyloid-beta. Unfortunately, in Alzheimer’s patients, LRP1 levels plummet drastically, and the remaining proteins often fall victim to the sticky amyloid-beta themselves.
Addressing this issue, Battaglia’s team crafted an intelligent delivery method using a tiny synthetic capsule termed a polymersome. This nanoparticle serves as a structured drug—a composite creating a methodical interaction with the blood-brain barrier.
“Our nanoparticles consist of numerous small components that connect, resembling building blocks. These components do more than just deliver medication; they smartly interact with the blood-brain barrier. This is why we refer to them as supramolecular drugs: they activate the brain’s own mechanisms that may have stalled due to Alzheimer’s,” Battaglia explains to ZME Science.
The sphere’s surface brims with special peptides acting as keys to the LRP1 receptacles. Yet, it isn’t solely about crafting these keys; the quantity is what matters most. This concept—multivalency—is absolutely crucial.
If there are too few keys, the particles would falter. Too many, however, would blur the line with sticky amyloid plaques, potentially causing issues. Ultimately, they affixed 40 keys per particle and proceeded to test on mice with significant amyloid-beta build-up, particularly a group bred for rapid Alzheimer’s development (APP/PS1 mice)—these older mice exhibited pronounced cognitive deterioration. They received one dose of the treatment.
Remarkably, by two hours post-injection, Aβ concentrations diminished by almost 45%.
Is This a Future Treatment?
The drop wasn’t just limited to the brain; increasing levels were observed in the bloodstream, marking a vital indication that the toxic plaques were indeed being expelled from the brain for elimination. The decrease in Aβ from the brain correlated perfectly with the excess found in the blood.
The intriguing part is how such results have lasted. Six months later, treated mice appeared unaffected by Aβ accumulation, seemingly free from adverse effects.
“The durability of the results is surprising. The rapid decline in amyloid levels indicates that once the barrier is operating effectively, the brain has the capacity to cleanse itself efficiently. Even months down the line, we are still witnessing improved memory and brain activity in treated subjects. It appears that kickstarting your brain’s own repair machinery yields lasting benefits rather than just passing ones,” notes Battaglia.
Indeed, as of now, Alzheimer’s still has no definitive cure, and the existing medicines can only slow its progression. None compare to the significant advances suggested by this study. Although there’s much work ahead, and this remains in early stages, the research holds promise.
“Significant tasks remain prior to human trials, but these results inspire strong optimism. Our forthcoming steps focus on further validating safety in broader preclinical models and rigorous toxicology assessments under regulatory compliance. If these align with our previous findings—prominent recovery without toxicity—we aim to transition towards initial clinical evaluations. Our overarching ambition is to translate this lab innovation into practical treatments, enabling new means to tackle Alzheimer’s by revitalizing the brain’s natural protections and restoration capabilities,” Battaglia concludes, offering a flicker of genuine hope—possibly a view into the future of Alzheimer’s treatment.
This narrative was first shared by ZME Science. Want to stay ahead in your quest for knowledge? Subscribe to our newsletter for the latest in science!