Our natural world can teach us a lot, especially when it comes to science. For nearly a century, researchers have been fascinated by how some mammals and birds can cope with harsh environments by slipping into a state called torpor. During this state, their body temperature and metabolism dip, which helps them conserve energy and heat.
In a recent study, Hong Chen, a professor specializing in biomedical engineering at the McKelvey School of Engineering, teamed up with an interdisciplinary group of experts to experiment with creating a similar torpor-like state in mice. They did this by using focused ultrasound to target a specific area in the brain known as the hypothalamus, which plays a key role in regulating body temperature and metabolism.
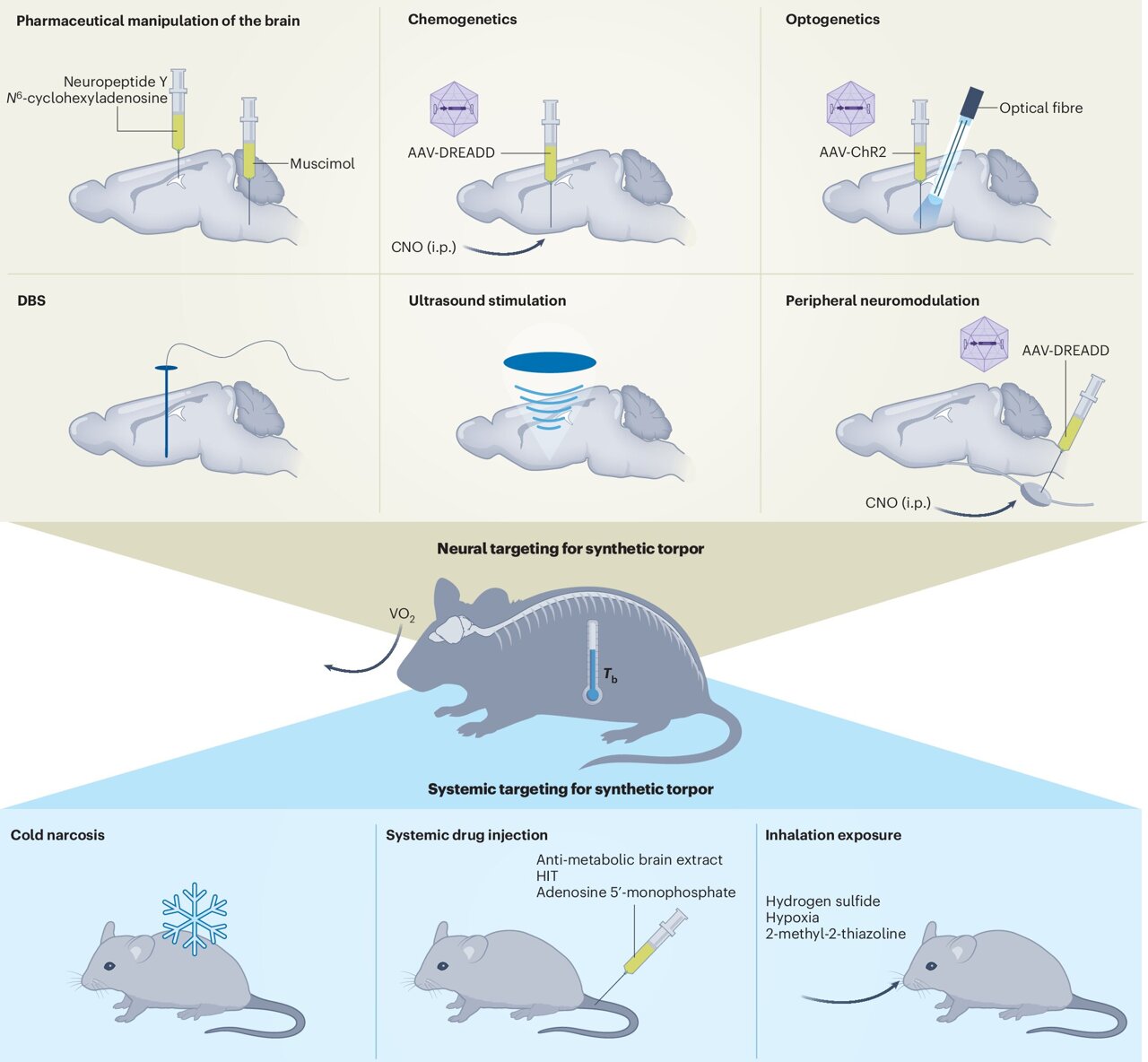
Interestingly, they didn’t just stop with mice, which naturally enter torpor; they also got rats, who don’t normally show this ability, to experience it. This research is documented in a Perspectives paper published in Nature Metabolism.
The team demonstrated a groundbreaking method of inducing a torpor-like state noninvasively by impacting the central nervous system – a first in the field.
Next up, they’re aiming to apply synthetic torpor techniques to benefit humans, particularly in situations where blood flow to vital organs is limited, like during surgeries or potentially when traveling through space to guard against radiation damage.
Unlike traditional medical practices that aim to enhance energy supply—like restoring blood flow post-stroke—synthetic torpor aims to decrease the body’s energy requirements instead.
Chen remarked, “Synthetic torpor’s ability to manage metabolism could genuinely change the game in medicine, introducing fresh avenues for treatment. “
While synthetic torpor has shown promise in animal experiments, bringing these methods into human medicine poses its own set of hurdles. A previous trial focusing on hydrogen sulfide was cut short due to safety concerns.
“We’re up against challenges like figuring out the metabolic differences between animals and humans, determining the right medication dosages, and designing ways for safely stepping in and out of this altered state,” said Wenbo Wu, a doctoral student working in Chen’s lab and the lead author of the study. Wu collaborated with Genshiro Sunagawa from the RIKEN Center for Biosystems Dynamics Research in Japan on this project.
He added that a combination of scientists, clinicians, and ethicists will play a vital role in developing safe and effective synthetic torpor methods for medical use.
Along with her team, which included now-assistant professor Yaoheng (Mack) Yang, Chen created a novel wearable device that uses ultrasound to stimulate neurons in the hypothalamus preoptic area. This stimulation could lower a mouse’s temperature by about 3 degrees Celsius for around an hour.
Moreover, during this period, the mice switched their energy sources from carbohydrates and fats to just fats, a crucial characteristic of entering torpor, while also experiencing a 47% decrease in heart rate—all under normal room temperature conditions.
Chen noted the unique nondestructive nature of ultrasound, saying, “It can safely penetrate the skull and target deep brain areas accurately — something that other methods cannot do. Even though it’s less specific for cell types compared to genetic methods, ultrasound offers an excellent noninvasive alternative for inducing synthetic torpor without genetic alterations. “
The potential therapeutic angles of synthetic torpor are exciting, from slowing tumor growth to developing treatments for tau protein-related diseases, like Alzheimer’s.
That said, there’s still a lot to explore regarding how different brain areas, organs, and cellular pathways nudge metabolic suppression and activation.
Researchers are pushing for further investigations to understand long-term risks and side effects, and they advocate for even more studies and innovations, which may involve tweaking neural circuits related to low metabolism and coordinating this with systemic treatments via medications or neural enhancements.
As Chen emphasizes, “Synthetic torpor isn’t just a pipe dream anymore; it’s an evolving space hungry to redefine medicine. Bridging neuroscience with bioengineering and practical medical applications is crucial for turning synthetic torpor from concept to reality through teamwork. ”
For more details: Wu, W., et al. “Synthetic torpor: advancing metabolic regulation for medical innovations”. Nature Metabolism (2025). DOI: 10.1038/s42255-025-01345-3doi.org/10.1038/s42255-025-01345-3
Content provided by Washington University in St. Louis.
Originally published by Medical Xpress.