Researchers at the University of Iowa have just hit a milestone in stopping craniosynostosis. This condition, which makes babies’ skulls fuse too soon, can lead to serious complications. Let’s break down what they’ve found.
Craniosynostosis occurs when the joints that connect a baby’s skull bones close early, which doesn’t leave room for their brain to grow properly. If not addressed through surgery, it can lead to abnormal head shapes and facial features. Evidence shows that this happens in about 1 in every 2,200 live births.
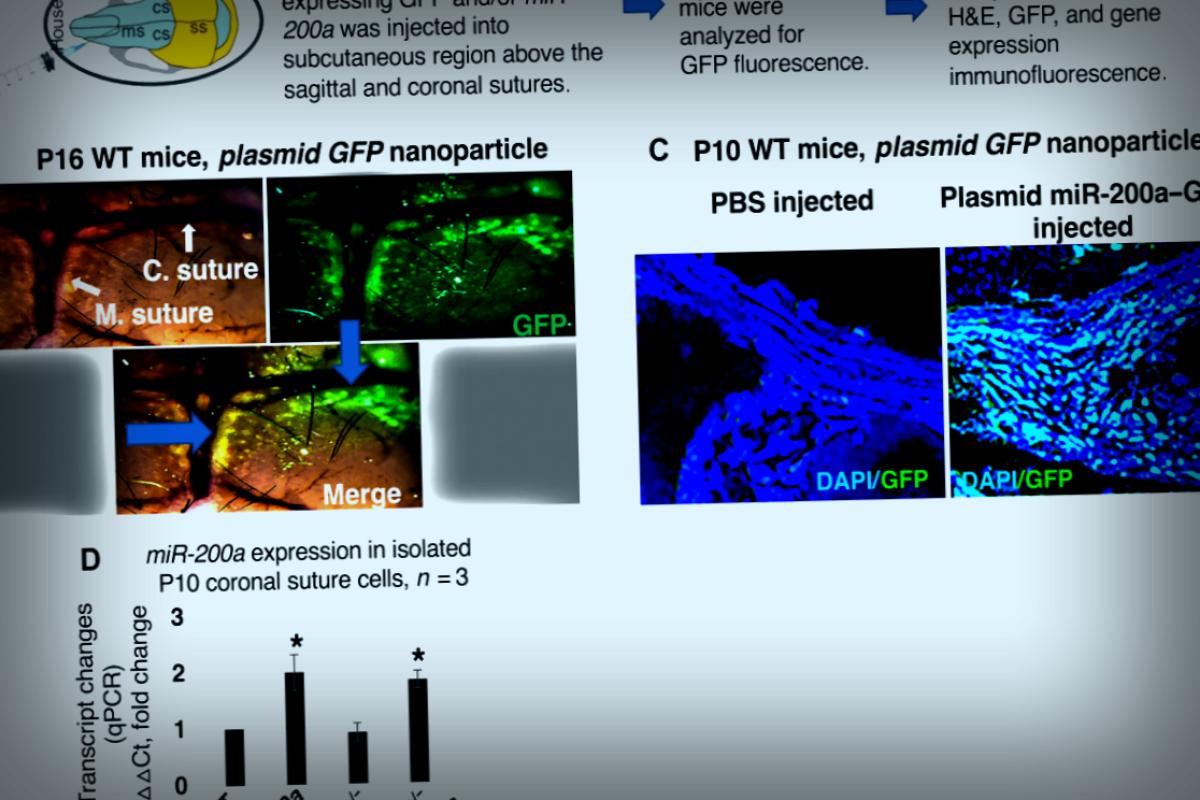
The research team dug into one specific gene, called miR-200a, crafting a tiny RNA nanoparticle that was injected just under the skin on the heads of newborn mice. These nano-packets found their way to the joints in the skull and released the miR-200a gene, leading to increased gene production. This gene stimulation kept the skull’s sutures from fusing, ensuring that the infant mice had enough space for their brains to develop properly.
As Professor Brad Amendt, who leads the study and is part of both the Anatomy and cell biology and Orthodontics departments, states, “This marks a significant first in gene therapy aimed at treating craniosynostosis. It opens up possibilities for better care without excessive trauma to children.”
The study titled Inhibition of craniosynostosis and premature suture fusion in Twist1 mutant mice with RNA nanoparticle gene therapy is published online in the journal Science Advances on August 22.
Interestingly, there are over 40 genes linked to craniosynostosis. However, Amendt decided to focus on miR-200a after realizing it played a key role in craniofacial development. His team tested this by blocking the gene in infant mice, who then displayed symptoms of craniosynostosis.
To tackle this challenge, amendments partnered with Professor Kevin Rice from the College of Pharmacy. They developed a 95-nanometer nanoparticle that carried the miR-200a gene. This small size made it easy for cells to engage with it as if welcoming a guest into their home.
Professor Amendt elaborates, “Cells have a mechanism where they recognize this nanoparticle, take it in, and then they can release the gene inside, allowing the cells to express it.”
In the researchers’ experiments, they injected these nano-packets just once into eight specially designed infant mice with craniosynostosis. Remarkably, none of them developed the condition, and the skull’s sutures remained open for a full 17 days, demonstrating the lasting impact of this gene therapy on brain growth.
According to Amendt, this system proved to be completely safe, showing no toxic effects on the skull or an increase in cancer risks. He leads the Craniofacial Anomalies Research Center at Iowa, spearheading this vital research.
Currently, detecting craniosynostosis before birth is challenging. However, once identified, the intervention is quite manageable. Surgeons typically perform a procedure known as endoscopic strip craniectomy, followed potentially by this gene treatment.
On a promising note, Amendt has already reached out to the U.S. Food and Drug Administration to begin testing this gene therapy on human infants, aiming to kick off clinical trials within the next couple of years.
As he puts it, “My focus is on improving patient health outcomes and exploring new ways to help patients recover,” making the prospect of this treatment all the more satisfying.
The lead author of this study, Samuel Swearson, conducted the crucial mice experiments as part of his Biomedical Engineering Graduate Program work, with other key contributors like Steve Eliason and Dan Su from Stanford University.
For more details: Samuel Swearson et al, Inhibition of craniosynostosis and premature suture fusion in Twist1 mutant mice with RNA nanoparticle gene therapy, Science Advances (2025). DOI: 10.1126/sciadv.adx9763
Provided by University of Iowa
This story originally appeared on Phys.org.