A fascinating breakthrough in Alzheimer’s research is making waves! A team from the Institute for Bioengineering of Catalonia (IBEC) and West China Hospital Sichuan University, along with their UK partners, has made significant strides using nanotechnology to counteract Alzheimer’s in mice.
This approach is quite a departure from traditional nanomedicine which commonly uses nanoparticles just to deliver drugs. Instead, these nanoparticles themselves act as active components, termed “supramolecular drugs.” Their findings have been detailed in the research journal Signal Transduction and Targeted Therapy.
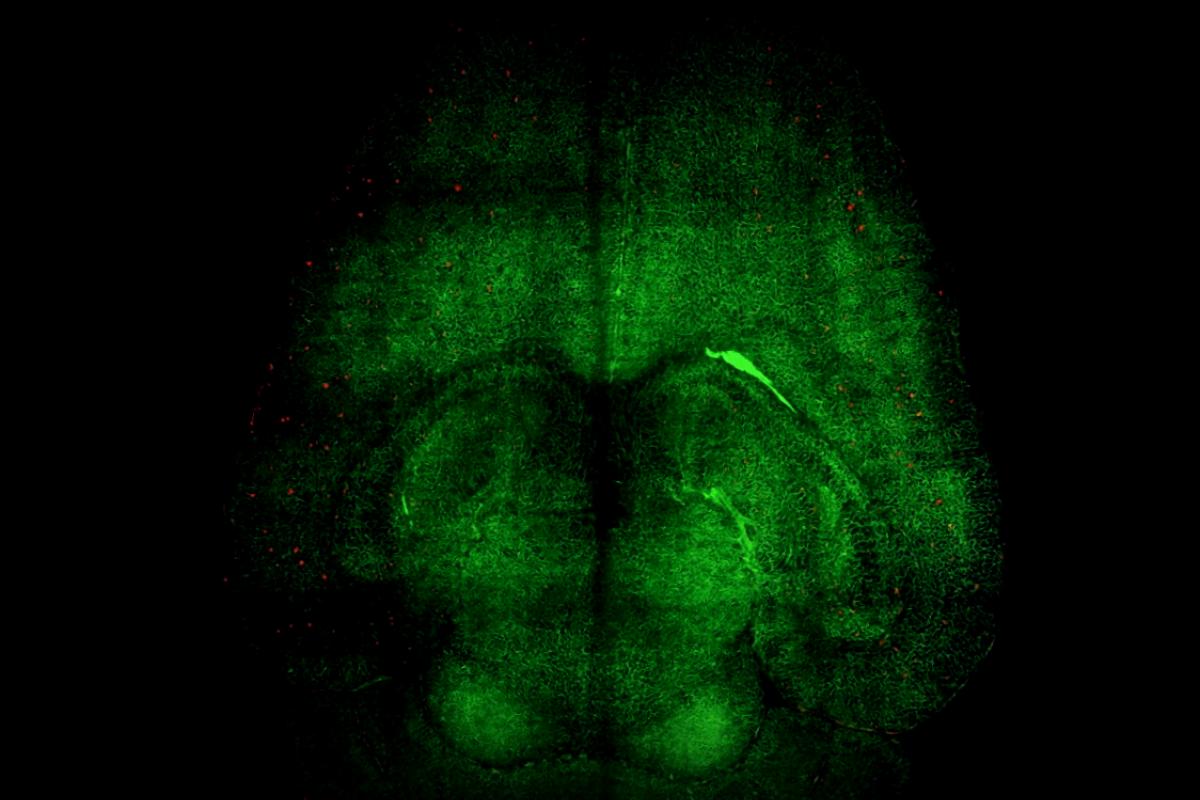
What makes this therapy stand out is its focus on restoring the blood-brain barrier (BBB), the crucial barrier that protects the brain, rather than directly targeting neurons. By enhancing the functionality of this barrier, researchers have reversed the pathological effects of Alzheimer’s in their animal subjects.
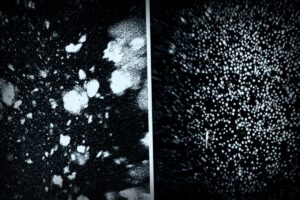
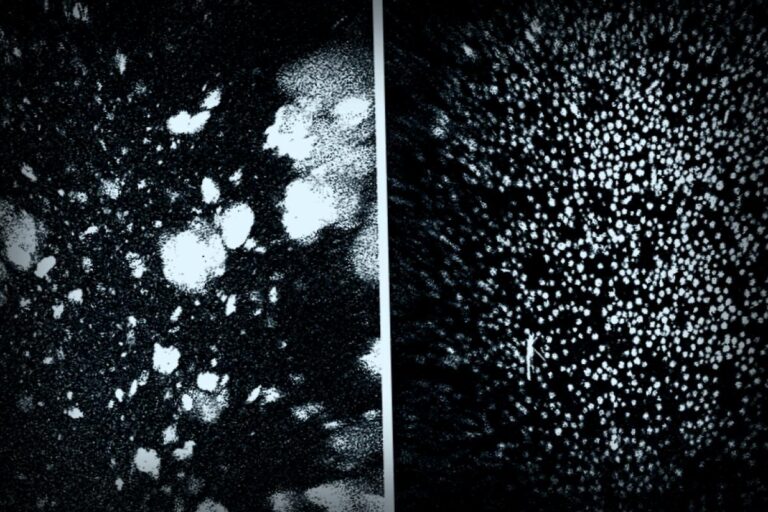
The blood-brain barrier is essential for shielding the brain from harmful pathogens or toxins that can enter through the bloodstream. This clever research targeted a specificmechanism that allowed harmful “waste proteins” to exit the brain through the BBB and enter the bloodstream for removal. For patients suffering from Alzheimer’s, the major culprits are proteins known as amyloid-β (Aβ), whose buildup negatively affects neurons.
Messing around with genetically modified mouse models that produced excess Aβ and started to show cognitive decline reminiscent of Alzheimer’s, researchers provided just three doses of their special supramolecular drugs, tracking the Alzheimer’s progression closely afterward.
Junyang Chen, the study’s first author and a researcher at the West China Hospital, noted the significant results, stating, “We saw a 50-60% reduction in Aβ levels in the brain just one hour post-injection.” The therapeutic outcomes were astounding too.
The researchers observed several animals over months, revealing their memory function and behavior at different disease stages. One striking experiment involved a 12-month-old mouse (similar to a 60-year-old human) treated with the nanoparticles. After six months, this mouse, which had aged into an 18-month-old adult (akin to a 90-year-old human), displayed behavior typical of healthy mice.
“We believe the long-lasting effects stem from the restoration of the brain’s vascular network,” said Giuseppe Battaglia, group leader in the study and ICREA Research Professor at IBEC. He explained that toxic proteins like Aβ trigger disease advancement, but improved vascular functions lead to clearing away these toxins undone, letting the whole brain recover balance.
Understanding Amyloid-β Removal
In light of cognitive decline from Alzheimer’s, it’s significant that the commonly inefficient system for clearing Aβ in also comes into play here. Under regular conditions, a protein called LRP1 works as a gatekeeper, helping move Aβ out of the brain through the bloodstream, but if it gets too overwhelmed, or not activated enough, Aβ starts to accumulate again, ensuing cognitive issues.
The cool supramolecular drugs from this team work by effectively triggering this gatekeeping system. By mimicking the elements LRP1 needs, they can help shuttle Aβ out of the brain, effectively restoring the vascular system’s role as a filtering pathway.
Therapeutic Innovations in Alzheimer’s Treatment
These nanoparticles are exciting not just for their role as vehicles for medications, but as full-fledged therapeutic agents. Crafted using a unique bottom-up molecular engineering method, they precisely regulate their size and surface properties, enhancing their interactions with cellular receptors. This specificity allows them to impact cell membrane activity more creatively, seamlessly improving the process of clearing out amyloid-β and replenishing essential blood circulation for sustaining healthy brain function.
This tantalizing new therapeutic route holds potential for tangible interventions for battling Alzheimer’s, giving hope that vascular contributions could elevate patient care outcomes. According to Lorena Ruiz Perez, another lead researcher, their results in facilitating rapid Aβ removal represent a significant concept shift: “Our findings indicate a remarkable capability to clear Aβ swiftly and restore normal functionality in the blood-brain barrier, reversing Alzheimer’s issues.”
This critical research effort gathered teams across institutions, including IBEC, West China Hospital Sichuan University, and University College London, among others.
For further reading: Check out the published research on Signal Transduction and Targeted Therapy (2025): Multivalent modulation of endothelial LRP1 induces fast neurovascular amyloid-β clearance and cognitive function improvement in Alzheimer’s disease models. DOI: 10.1038/s41392-025-02426-1
This research comes to light thanks to the Institute for Bioengineering of Catalonia.